Population density cycles of small rodents create a boom and bust system driving the dynamics of the tundra food web, both in their functions as prey and as consumers. Warmer and wetter winters are expected to hamper rodents access to food, resulting in dampened, irregular or lost rodent population cycles. How will these changes cascade through the food web?
Small rodents in tundra foodwebs
Small rodents are a key component of the low arctic tundra ecosystem at Varanger. Svalbard lacks native small rodents, but has a local population of an introduced vole species (the Sibling voles) that shares a dangerous (zoonotic) parasite with the Arctic fox. Population cycles of arctic small rodents have typically a period of three to five years, but there is considerable variation in cycle period and amplitude. During the last decades, small rodent cycles have been fading out in several localities in the Arctic.
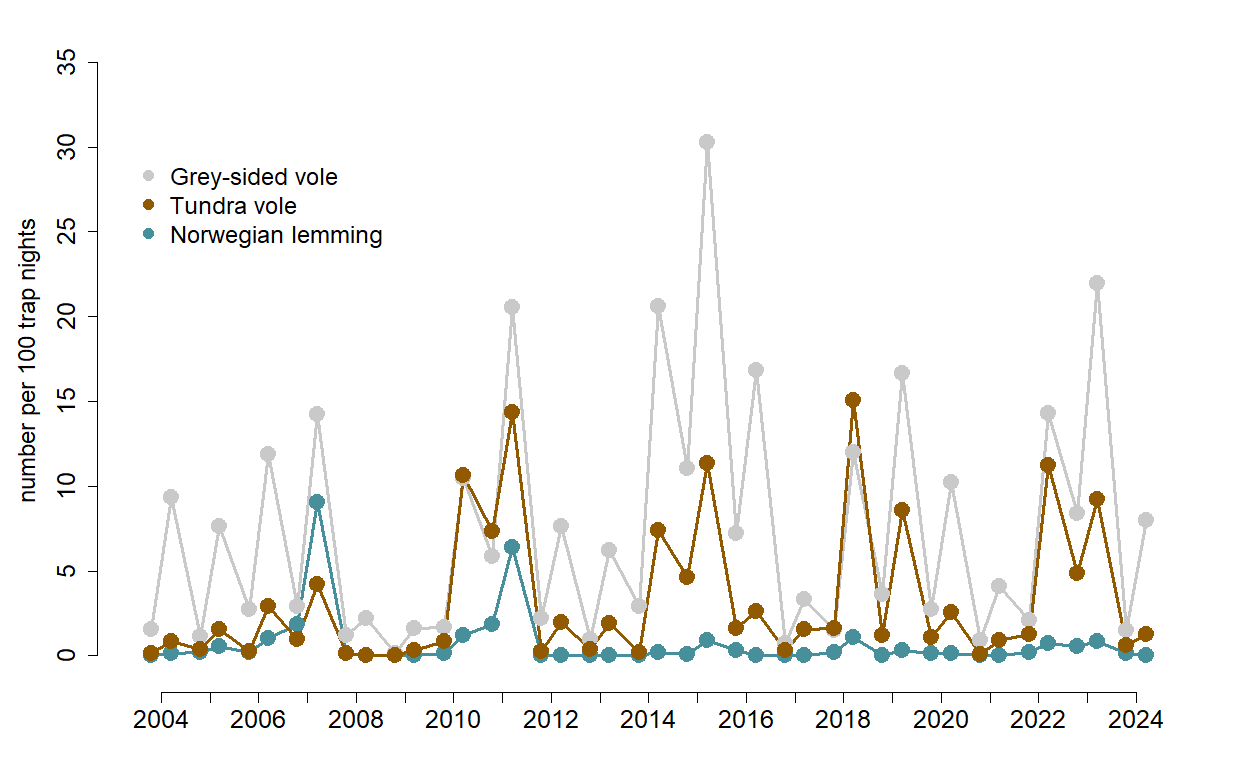 Population time series of three most common rodent species in Varanger Peninsula the Norwegian lemmings, Grey-sided voles and Tundra voles. Note that although the species are synchronized their cycles have different amplitudes, shapes and regularity (i.e. the lemming is missing in the last peak in 2015).
Population time series of three most common rodent species in Varanger Peninsula the Norwegian lemmings, Grey-sided voles and Tundra voles. Note that although the species are synchronized their cycles have different amplitudes, shapes and regularity (i.e. the lemming is missing in the last peak in 2015).
The population density cycles create a boom and bust system driving the dynamics of the tundra food web, both in their functions as prey and as consumers. The three rodent species (figure to the right) have very different diets, different sensitivity to climate and value to predators. It is therefore important to consider them separately in many respects.
- All tundra carnivores generally consume small rodents. Rodents are, however, particularly important as a subsistence prey for several specialist predators, such as arctic fox, stoat, least weasel, long-tailed skua, rough-legged buzzard and snowy owl. Lemmings have a larger impact than voles on some predators, especially arctic fox and snowy owl. These predators have high reproductive output during the peaks of the cycles, while they may skip breeding entirely during the lows. Several generalist predators, such as red fox and corvids, also respond to the rodent cycle. Their increased abundance causes affects also other prey species such as ptarmigan (so called alternative prey).
- During peak years, rodents consume vast quantities of plant foods. This affects both the biomass and species composition of plant communities. Small rodent module addresses these interactions in heaths and snow bed habitats. The effect of tundra voles on tall shrub encroachment is addressed in the Tall Shrub module.
Expected climate impact
Climate change is expected to lead to warmer and wetter winters. This will lead to increased proportion of precipitation coming as rain instead of snow, leading to melting-freezing events and ice crust formation. Icy snow limits the rodents, especially lemmings, access to plant foods. Dampened, irregular or lost rodent population cycles are a likely result. Sensitivity of lemmings may render the rodent guild dominated by voles.
These changes are predicted to have cascading impacts in the ecosystem. Boreal generalist predators will replace the lemming dependent arctic predators. Reduced rodent herbivory will contribute to vegetation state changes.
Expected effects of climate change on tundra food web through small rodents. The direct impact of winter climate on the rodents populations are considered most important. We also expect an indirect effect of climate through its effect on plant communities’ function as food and cover for the rodents. Generalist predators’ abundance is also determined by availability of ungulate prey and ungulate management. Besides the tree rodent species small rodent module focuses on climate responses of the plant communities tundra heaths and snow beds and the specialist predators least weasel, stoat, long-tailed skua, rough-legged buzzard and snowy owl.
Management relevance
- Information about the population dynamics of rodents (and in particular lemmings) are important when deciding on the when and where to allocate management actions to support populations of red listed arctic predators such as the Arctic fox .
- Rodent population dynamics have indirect effects on small game, like ptarmigan. Information about rodent abundance can therefore aid forecasting game population dynamics and devising efficient game harvesting strategies.
Monitoring methods
Rodents: Year-round camera monitoring in an extensive system of permanent camera boxes (established gradually 2015-2021). Snap trapping (summer and fall, gradually replaced by camera trapping) and fecal counts (summer) with existing time-series since 2005.
Plant communities: In heaths, annual maximum biomass of all functional plant groups in tundra heaths based on point intercept frequency (time-series since 2005). In snowbeds, occurrence of dwarf shrubs in permanent plots (time-series since 2009), and snowbed extent (time-series since 2021). To separate the effect of climate change, rodents, and ungulates on plant communities, exclosures of rodents and ungulates were established in 2019-2021 in snowbed, heath, and meadow habitats (in collaboration with the Tall-shrub module).
Predators: Least weasel and stoat abundance by small mammal camera traps (see rodents). Rough-legged buzzards and snowy owls breeding population density by visits to traditional nest sites. Long-tailed skua breeding population density by territory counts in breeding habitats and demography by means of capture-resighting of ringed birds.